An atom with an atomic mass of 89 and an atomic number of 39 has how many neutrons
The correct answer is 50
Explanation:
The atom is known as the smallest unit of matter, this is composed of a nucleus with neutrons and protons (particles positively charged), as well as electrons (particle negatively charged) that are outward the nucleus. In this context the atomic number of an atom is the same that the number of protons which is unique to each element, this means the atom of the question contains 39 protons as the atomic number is equivalent to 39; in the same way, the atomic mass refers to the sum of protons plus neutrons that in this case is 89.
According to this, the number of neutrons can be found by subtracting the number of protons expressed in the atomic number from the atomic mass that includes both the protons and the neutron which is shown below.
89 (number of protons and neutrons) - 39 (number of protons) = 50 neutrons
Explanation:
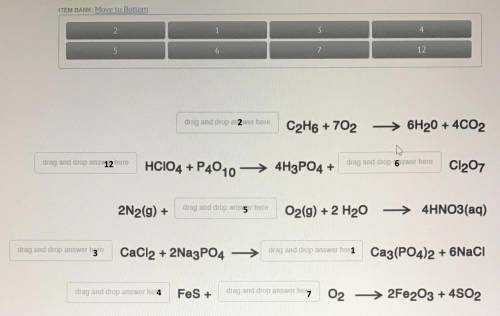
Balance Chemical Equation:
A balanced chemical equation is that in which the the number of the reactant atoms equal to the number of the product atom.
For example if the oxygen at the reactant side is 4, the number of oxygen should be 4 on the product side.
The complete answer is in attachment
Equation 1:
2 is the correct number
In the equation look for the number of Carbon on product side
C = 4 so it should be 4 on reactant side too
So,
by putting 2 in Coefficient location the number of C become equal on reactant as well as product side.
____________________
Equation 2:
12 on reactant side and 6 on product side is the correct number
In the equation look for the number of Hydrogen (H) on product side
it is H = 12 so it should be 12 on reactant side too
Then look for the number of Chlorine (Cl) on reactant side
C = 12 so it should be 12 on Product side too
So,
by putting 12 in Coefficient location of the reactant side and 6 on product side the number of H and Cl become equal on reactant as well as product side.
______________________
Equation 3:
5 is the correct number
In the equation look for the number of Oxygen on product side
O = 12 so it should be 12 on reactant side too
So,
by putting 5 in Coefficient location the number of O become equal on reactant as well as product side.
______________________
Equation 4:
3 on reactant side and 1 on product side is the correct number
In the equation look for the number of Chlorine (Cl) on product side
it is Cl = 6 so it should be 6 on reactant side too
Then look for the number of Calcium (Ca) on reactant side
Ca = 1 so it should be 1 on reactant side too
So,
by putting 3 in Coefficient location of the reactant side and 1 on product side the number of Cl and Ca become equal on reactant as well as product side.
____________________
Equation 5:
4 and 7 on reactant side is the correct number
In the equation look for the number of Sulfur (S) on product side
it is S = 4 so it should be 4 on reactant side too
Then look for the number of Oxygen (O) on product side
O = 14 so it should be 14 on reactant side too
So,
by putting 4 and 7 in Coefficient location of the reactant side so the number of S and O become equal on reactant as well as product side.
Popular Questions about the subject: Chemistry
New questions by subject
from an AI-bot






