Chemistry
22.07.2020 13:02
113
400
7
Solved by an expert
Ahydrogen-like ion is an ion containing only one electron. the energy of the electron
Ahydrogen-like ion is an ion containing only one electron. the energy of the electron in a hydrogen-like ion is given by en = ? (2.18 × 10? 18j) z2 ( 1 n2 ) where n is the principal quantum number and z is the atomic number of the element. plasma is a state of matter consisting of positive gaseous ions and electrons. in the plasma state, a mercury atom could be stripped of its 80 electrons and therefore could exist as hg80+. use the equation above to calculate the energy required for the last ionization step: hg79+(g) ? hg80+(g)+ e?
Show Answers
samueltaye
4,8(23 marks)
Energy required for the ionization of mercury is 
Explanation: Energy of the electron in a hydrogen-like ion is given by the equation:
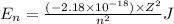
where, Z = atomic number
n = principle quantum number
Ionization energy is the energy required to release the outermost electron from an isolated gaseous atom.
For the ionization of mercury, the equation follows:
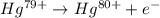
Mercury has an atomic number = 80
As, in this element 79 protons are already released, which means that the electronic configuration for 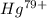 is
is 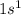
and the principle quantum number for the last ionization step = 1
Putting the values in energy equation, we get
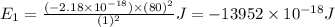
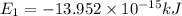 (Conversion Factor: 1kJ = 1000J)
(Conversion Factor: 1kJ = 1000J)
emj70
4,6(17 marks)
Silicon
I hope this helps you
Popular Questions about the subject: Chemistry
10 mol/h of 50 mol % NaOH is mixed with enough 6.5 mol % NaOH to produce...
Chemistry
28.10.2022 16:20
Determine the number of particles in 0.332 g of NaCl (molar mass NaCl=58.44...
Chemistry
15.01.2023 03:41
Match each type of sedimentary rock with its composition. 1. mineral crystals...
Chemistry
23.10.2020 01:04
Titanium dioxide (TiO ) is used extensively as a white pigment. It is produced...
Chemistry
21.09.2020 23:15
In a constant‑pressure calorimeter, 70.0 mL of 0.350 M Ba(OH)2 was added...
Chemistry
22.08.2021 01:00
How many moles of argon are present if there are 1.23 x 10^20 atoms?...
Chemistry
04.01.2020 00:00
If the electronegativity of H is 2.1 and of Cl is 3.5, which type of bond...
Chemistry
27.06.2021 09:57
How many grams are in 3.35 moles Cu2CO3? show your work...
Chemistry
07.10.2022 10:12
Determine the mass of CO2 formed if 17 grams of C4H10...
Chemistry
08.02.2022 03:16
How many moles of KOH are in 855g KOH? show your work...
Chemistry
29.01.2023 11:30
New questions by subject
The fact that each branch of government must be separate from the others...
Mathematics
09.09.2022 21:23
Which sentence about subject-verb agreement is not true? singular subjects...
English
27.01.2023 09:33
With a dui charge on a drivers record a. the price of his or her insurance...
Health
29.01.2020 00:42
Summarize this excerpt from the necklace in one sentence....
English
30.03.2023 14:22
Why is te word quarantine used intead of blockade...
History
15.02.2020 13:40
Transform both equations in each system of equations so that each coefficient...
Mathematics
16.11.2021 23:04
Liam is a hacker who tries to access wi-fi signals while driving his car....
Computers and Technology
12.07.2021 09:17
How to use maps and other geographic representations, tools, and technologies...
Geography
12.03.2020 18:28
Stream of consciousness is a writing technique in which: a.the writer s thoughts...
English
15.12.2022 18:32
The number cubes the fifth grade math classes use are packed into a box....
Mathematics
25.02.2021 06:02
Find out answers to questions
from an AI-bot
from an AI-bot
Get full access
Answers






