10 mol/h of 50 mol % NaOH is mixed with enough 6.5 mol % NaOH to produce a 10 mol
105.4 kJ
Explanation:
Given that:
10 mol/h of 50 mol % NaOH is mixed with enough 6.5 mol % NaOH to produce a 10 mol % aqueous NaOH solution
Now,if the moles of 6.5 mol% of NaOH is mixed with 10 mol of 50% NaOH
We have; Total moles = (M + 10) moles
moles of NaOH = ( 10 × 0.5) + ( M × 0.065)
The mixture then tends to be the the mole of NaOH solution
= 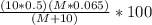
= 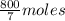
The total moles = 10 + 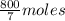
= 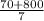
= 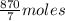
10% mole of NaOH & 90% moles of H₂O
Thus, 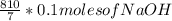
= 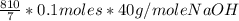
= 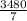 g of NaOH
g of NaOH
For H₂O
= 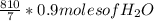
= 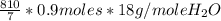
= 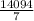 g of H₂O
g of H₂O
Finally, Total mass of mixture = 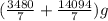
= 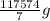
Heat capacity of solution = 2.8 J/g ° C
At 25 ° C, heat removed to change the temperature from 25° C to 10° C
ΔT = ( 25 - 10 ) ° C
ΔT = 15 ° C
ΔQ = mcΔT
ΔQ = 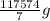 * 2.8 J/g ° C * 15° C
* 2.8 J/g ° C * 15° C
ΔQ = 105444 J
ΔQ = 105.4 kJ
Hence, the amount of heat to be removed to keep the final solution at 10 ° C = 105.4 kJ
See explanation
Explanation:
Let us consider the two compounds carefully. Carbon dioxide is purely a non-polar molecular compound while the bond between the C2^2- ion and Ca^2+ is ionic thereby making CaC2 an ionic compound.
Recall that ionic compounds have high melting and boiling points due to strong ionic interactions in the structure of the compound.
On the other hand, CO2 is a non-polar substance whose molecules are held together only by weak dispersion forces.
As a result of the reasons outlined above, carbon dioxide has a low boiling point and calcium carbide has a high boiling point.
Popular Questions about the subject: Chemistry
New questions by subject
from an AI-bot






