Chemistry
28.05.2022 23:46
261
404
5
Solved by an expert
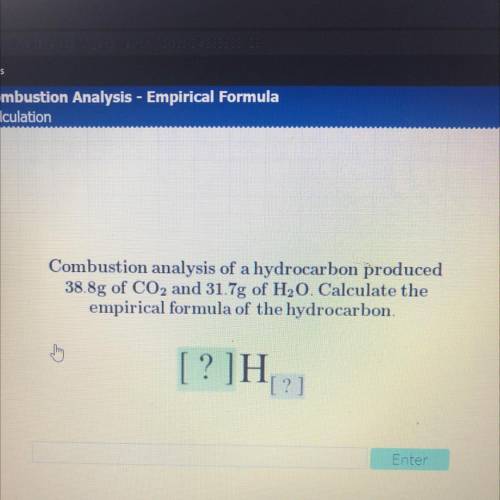
Combustion analysis of a hydrocarbon produced 38.8g of CO2 and 31.7g of H20. Calculate
Combustion analysis of a hydrocarbon produced
38.8g of CO2 and 31.7g of H20. Calculate the
empirical formula of the hydrocarbon.
[?]H
[?]
Show Answers
empirical formula of the hydrocarbon.
[?]H
[?]

tbiles99
4,5(35 marks)
Single bond shares one electron from each atom. Hence 2 electrons are shared.
Popular Questions about the subject: Chemistry
A copper wire (density = 8.96 ) has a diameter of 0.25 mm. If a sample...
Chemistry
15.11.2022 07:06
a 72 kg skydriver drops from a helicopter and is accelerating downwards...
Chemistry
05.07.2021 19:36
What energy is required to boil 2.15 moles of water at the boiling point...
Chemistry
02.08.2021 02:45
If it takes 46 mL of a 0.41 M of H2SO4 to neutralize 315 mL of an KOH...
Chemistry
09.09.2022 20:10
A jar is filled to the top with water, and a piece of cardboard is slid...
Chemistry
27.09.2020 00:51
I have oxygen gas at 2 L and 2 atm. At what pressure is the oxygen gas...
Chemistry
23.06.2020 10:22
¿QUE DATO DE LA CONFIGURACIÓN ELECTRÓNICA TE PERMITE UBICAR LOS ELEMENTOS...
Chemistry
27.10.2022 02:39
Which method do you think the suspects used to make the coins, additive...
Chemistry
08.01.2023 08:17
¿A qué se refiere cuando se dice que el trabajo científico es cualitativo...
Chemistry
18.05.2020 03:18
New questions by subject
Mm co. uses corrugated cardboard to ship its product to customers. management...
Business
02.10.2021 01:06
Lyon co. collected $1200 in sales tax from customers during the month...
Business
20.10.2021 17:21
andrea, a sales executive, believes that she has the competence to achieve...
Social Studies
10.07.2020 08:59
Msi is considering eliminating a product from its toddletown tours collection....
Business
12.05.2020 14:52
What was the relationship between the bloc républicain and the church,...
History
09.12.2022 03:48
Mammograms many women choose to have annual mammograms to screen for breast...
Health
01.06.2022 16:03
Make a list of women s shoes that are yellow, orange, or red. show three...
Computers and Technology
05.01.2022 18:17
What is an artistic triumph of the middle ages...
Social Studies
08.12.2021 02:06
Many gays and lesbians believe that their inability to marry legally is...
English
30.12.2022 00:37
Mnop inc. declared a $1.00 dividend with a record date of thursday, september...
Social Studies
14.12.2022 01:04
Find out answers to questions
from an AI-bot
from an AI-bot
Get full access
Answers






