Your doorbell is malfunctioning, It will only strike the bell one time when the
Electromagnet plays a very important role by creating a magnetic field which will in turn pull the armature towards it, thereby causing the hammer to strike the bell.
Explanation:
An electromagnet is simply a type of magnet where the magnetic field is produced with the help of an electric current.
In an electric bell, an electromagnet forms a very vital part alongside the armature, armature rod, spring, hammer and a gong.
Now, power that produces electric current in the electric bell is gotten when the switch for the bell is turned on.
However, hen the power that produces the electric current for the electromagnet stops, the magnetic field will stop being produced but when electric current is flowing through the coils, the electromagnet will create a magnetic field which will in turn pull the armature towards it, thereby causing the hammer to strike the bell.
Approximately 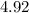 .
.
Explanation:
Initial volume of the solution: 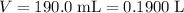 .
.
Initial quantity of 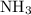 :
:
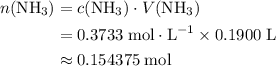 .
.
Ammonia 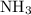 reacts with hydrochloric
reacts with hydrochloric 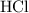 acid at a one-to-one ratio:
acid at a one-to-one ratio:
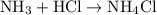 .
.
Hence, approximately 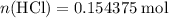 of
of 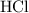 molecules would be required to exactly react with the
molecules would be required to exactly react with the 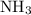 in the original solution and hence reach the equivalence point of this titration.
in the original solution and hence reach the equivalence point of this titration.
Calculate the volume of that 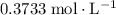
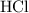 solution required for reaching the equivalence point of this titration:
solution required for reaching the equivalence point of this titration:
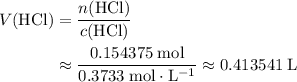 .
.
Hence, by the assumption stated in the question, the volume of the solution at the equivalence point would be approximately 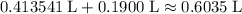 .
.
If no hydrolysis took place, 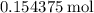 of
of 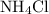 would be produced. Because
would be produced. Because 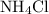 is a soluble salt, the solution would contain
is a soluble salt, the solution would contain 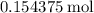 of
of 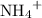 ions. The concentration of
ions. The concentration of 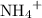 would be approximately:
would be approximately:
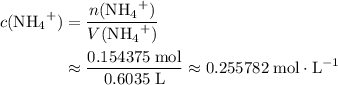 .
.
However, because 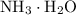 is a weak base, its conjugate
is a weak base, its conjugate 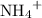 would be a weak base.
would be a weak base.
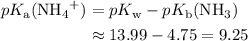 .
.
Hence, the following reversible reaction would be take place in the solution at the equivalence point:
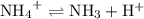 .
.
Let 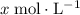 be the increase in the concentration of
be the increase in the concentration of 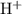 in this solution because of this reversible reaction. (Notice that
in this solution because of this reversible reaction. (Notice that 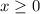 .) Construct the following
.) Construct the following 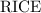 table:
table:
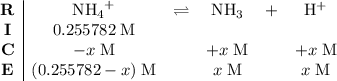 .
.
Thus, at equilibrium:
Concentration of the weak acid:![[{\rm {NH_4}^{+}}] \approx (0.255782 - x) \; \rm M](/tpl/images/1135/8062/3a08e.png) .Concentration of the conjugate of the weak acid:
.Concentration of the conjugate of the weak acid: ![[{\rm NH_3}] = x\; \rm M](/tpl/images/1135/8062/5c451.png) .Concentration of
.Concentration of 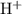 :
: ![[{\rm {H}^{+}}] \approx x\; \rm M](/tpl/images/1135/8062/2a607.png) .
.![\displaystyle \frac{[{\rm NH_3}] \cdot [{\rm H^{+}}]}{[{ \rm {NH_4}^{+}}]} = 10^{pK_\text{a}({\rm {NH_4}^{+}})}](/tpl/images/1135/8062/50d33.png) .
.
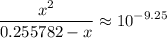
Solve for  . (Notice that the value of
. (Notice that the value of  is likely to be much smaller than
is likely to be much smaller than 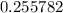 . Hence, the denominator on the left-hand side
. Hence, the denominator on the left-hand side 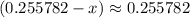 .)
.)
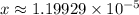 .
.
Hence, the concentration of 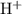 at the equivalence point of this titration would be approximately
at the equivalence point of this titration would be approximately  .
.
Hence, the 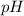 at the equivalence point of this titration would be:
at the equivalence point of this titration would be:
![\begin{aligned}pH &= -\log_{10}[{\rm {H}^{+}}] \\ &\approx -\log_{10} \left(1.19929 \times 10^{-5}\right) \approx 4.92\end{aligned}](/tpl/images/1135/8062/8a0d9.png) .
.
Popular Questions about the subject: Chemistry
New questions by subject
from an AI-bot






