Which conclusion can be made about isotopes? 1.isotopes that are naturally occurring
3.isotopes with more neutrons than protons are always unstable
4.isotopes with the same number of neutrons as protons are always the most abundant
4.999 moles of excess reactant will be left over.
Explanation:
Limiting reagent is defined as the reagent which is completely consumed in the reaction and limits the formation of the product.
Excess reagent is defined as the reagent which is left behind after the completion of the reaction.
The number of moles is defined as the ratio of the mass of a substance to its molar mass.
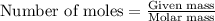 .....(1)
.....(1)
Given mass of aluminium carbide = 112 g
Molar mass of aluminium carbide = 143.96 g/mol
Putting values in equation 1:
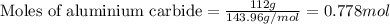
For the given chemical reaction:
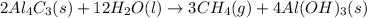
By the stoichiometry of the reaction:
2 moles of aluminium carbide reacts with 12 moles of water
So, 0.778 moles of aluminium carbide will react with = 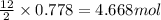 of water
of water
Given mass of water = 174 g
Molar mass of water = 18 g/mol
Putting values in equation 1:
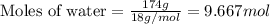
Moles of excess reactant (water) left = 9.667 - 4.668 = 4.999 moles
Hence, 4.999 moles of excess reactant will be left over.
Popular Questions about the subject: Chemistry
New questions by subject
from an AI-bot






