Chemistry
24.06.2022 14:35
151
464
9
Solved by an expert
When the protein gramicidin is integrated into a membrane, an h channel forms and
When the protein gramicidin is integrated into a membrane, an h channel forms and the membrane becomes very permeable to protons (h ions). if gramicidin is added to an actively respiring muscle cell, how would it affect the rates of electron transport, proton pumping, and atp synthesis in oxidative phosphorylation
Show Answers
mez64
4,8(25 marks)
0.72 moles of hydrochloric acid are needed to completely react with 0.36 mol of lead.
Explanation:
The balanced reaction is:
Pb + 2 HCl → PbCl₂ + H₂
By stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of reagent participate:
Pb: 1 moleHCl: 2 molesYou can apply the following rule of three: if by stoichiometry of the reaction 1 mole of Pb reacts with 2 moles of HCl, 0.36 moles of Pb will react with how many moles of HCl?
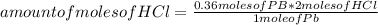
amount of moles of HCl= 0.72 moles
0.72 moles of hydrochloric acid are needed to completely react with 0.36 mol of lead.
Popular Questions about the subject: Chemistry
Calculate the number of atoms in a 7.45x10^3 g sample of sodium...
Chemistry
22.05.2021 00:43
How many atoms of phosphorus are in 8.50 mol of copper (ii) phosphate?...
Chemistry
03.04.2022 08:37
Need help fo #4 . Show me all the work and I’ll mark you brainliest...
Chemistry
06.07.2022 15:05
Which statement describes copper? O A. Copper is an insulator...
Chemistry
14.12.2021 13:29
(60 Points)If a student drops a 2.3 g piece of magnesium into...
Chemistry
04.03.2023 09:22
A 900.0 g sample of sea water is found to contain 3.5 x 10^-3...
Chemistry
04.06.2021 05:13
At high temperatures, carbon reacts with O2 to produce CO as follows:...
Chemistry
17.01.2021 15:55
3) A saturated solution of PbCl2 in water was prepared and filtered....
Chemistry
25.07.2022 17:27
Skill-F take naphthalene balls and wrap them in a piece of cloth....
Chemistry
23.04.2022 13:47
A buffer solution contains 0.100 M fluoride ions and 0.126 M hydrogen...
Chemistry
23.05.2021 15:32
New questions by subject
4.00 mL of a 1.50e-3 M solution of crystal violet is mixed with...
Chemistry
01.05.2021 23:54
A step test of a transducer brings on a damped oscillation decaying...
Engineering
02.08.2020 12:04
Calculate the molarity of a solution of KOH if 18.15 mL of it...
Chemistry
13.10.2020 10:46
The sensitivity range for an objective function coefficient is...
Computers and Technology
25.07.2022 15:51
Planes T and X are parallel. Plane T contains line a. Plane X...
Mathematics
07.10.2021 15:42
Solve the problem. A vendor sells hot dogs and bags of potato...
Mathematics
15.04.2020 15:23
WILL MARK BRAINLEST IF RIGHT Suppose that Hannah wants to find...
Social Studies
14.06.2022 03:03
Marissa bought 4 cans of corn and 8 cans of green beans for a...
Mathematics
14.04.2020 01:51
Your favorite team has made the playoffs, and you decide that...
Physics
21.05.2023 10:57
Find out answers to questions
from an AI-bot
from an AI-bot
Get full access
Answers






