Physics
22.07.2020 19:28
298
461
6
Solved by an expert
What is the change in internal energy (deltaE ) of a system when it loses 76.0 J
What is the change in internal energy (deltaE ) of a system when it loses 76.0 J of heat while the surroundings perform 29.0 J of work
Show Answers
villafana36
4,8(28 marks)
-47 J
Explanation:
Given that,
Heat loss = -76 J (negative for loss)
Work done by the surroundings = -29 J
We need to find the change in internal energy of a system.
The first law of thermodynamics is given by :
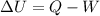
W is work done by the system
Putting all the values,
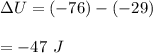
Hence, the change in internal energy is -47 J.
25cotahai
4,7(69 marks)
B. A single proton has +1 charge.
*I'm pretty sure*
*I'm pretty sure*
Popular Questions about the subject: Physics
Electromagnetic induction Paragraph...
Physics
24.08.2022 01:08
Calculate the wave number for the infrared absorption of D35Cl when...
Physics
24.11.2022 15:23
If 20N force produces an acceleration of 5ms^-2 In a body then the...
Physics
06.02.2020 11:22
How do you format short quotations in an MLA-style document?...
Physics
27.11.2022 16:59
he first law of thermodynamics states that the change U in the internal...
Physics
01.02.2022 00:05
As the velocity of your spaceship increases, you would observe Group...
Physics
02.07.2021 22:58
You are asked to design spring bumpers for the walls of a parking...
Physics
17.05.2022 16:25
Podrian darme un resumen de la historia del voleybol en venezuela...
Physics
07.12.2022 23:15
The generator at a power plant produces AC at 24,000 V. A transformer...
Physics
25.07.2021 04:26
A cannon launches a 3.0 kg pumpkin with 110 J of kinetic energy. What...
Physics
30.08.2022 06:45
New questions by subject
Can someone please tell me what this means? I have an essay to write...
English
22.06.2022 03:46
Which of the following best describes why the Sumerians needed more...
Social Studies
14.09.2022 15:45
I got 600 extra points who want it i need to know first who is the...
Mathematics
21.07.2022 09:19
Go where you may, search where you will, roam through all the monarchies...
English
25.09.2021 22:48
Why do people celebrate christmas?...
English
27.08.2020 05:17
Write an integer to represent $8 withdrawn from a bank account. A....
Mathematics
24.08.2020 21:41
Which sentence from the story provides a reflection of the Indian...
English
25.07.2021 21:12
The level of the elevation zone pyramid above that is labeled with...
Geography
24.06.2022 01:57
Which person has both potential and kinetic energy A. a person sitting...
Physics
27.05.2021 09:22
Find out answers to questions
from an AI-bot
from an AI-bot
Get full access
Answers






