Chemistry
04.05.2022 23:17
135
332
7
Solved by an expert
Then, calculate the molarity of a solution made by adding 5.57 g of cacl2 to enough
Then, calculate the molarity of a solution made by adding 5.57 g of cacl2 to enough water to create a solution with a total volume of 2.50 l. enter only a numerical value in the correct number of significant figures.
Show Answers
ethan5738
4,4(63 marks)
The answer to your question is: 0.02 M
Explanation:
Data
mass = 5.57 g
volume = 2.50 L
Formula
Molarity = 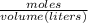
Process
1.- Calculate the molecular mass of CaCl₂
Molecular mass 0f CaCl₂ = 40 + (35.5 x 2) = 111 g
2.- Calculate the moles of CaCl₂ in 5.57 g
111 g of CaCl₂ ---------------- 1 mol
5.57 g of CaCl₂ ------------- x
x = (5.57 x 1) / 111
x = 0.050 moles of CaCl₂
3.- Calculate molarity
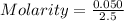
Molarity = 0.02
velazquezemmy45
4,5(42 marks)
D. divide 4.56 by molar mass of mg(OH)2
Popular Questions about the subject: Chemistry
How can you use these things in real life Atomic Theory Periodic...
Chemistry
20.12.2021 19:33
All Parts of Ubiquinone Have a Function In electron transfer,...
Chemistry
15.10.2022 05:41
Heeeellllllllp meeee please please help me...
Chemistry
28.12.2022 08:08
A feed of 10,000 kg/h is 10 wt% acetic acid in water. The acetic...
Chemistry
30.05.2020 23:03
How many liters are in a cubic meter? 100,000 100 10,000 1,000...
Chemistry
10.05.2020 03:01
3.) a 208 g sample of sodium-24 decays to 13.0 g of sodium-24...
Chemistry
30.10.2021 11:46
In utah s arches national park we can see many interesting shapes...
Chemistry
01.04.2020 18:17
What connection does the author draw between clouds and weather...
Chemistry
07.05.2023 14:25
How many atoms would be contained in 454 grams of iron (Fey?...
Chemistry
29.05.2021 10:21
A beaker contains 214.2 grams osmium (III) fluoride in 0.0673...
Chemistry
16.01.2022 19:42
New questions by subject
In which of the following situations will helper T cells be...
Biology
04.09.2022 22:31
Calculate the federal tax rate. The federal tax rate is a function...
Business
19.10.2022 20:25
A XY kg block of iron casting at 450 K is thrown into a large...
Chemistry
28.03.2021 01:29
Un gas se encuentra en un envase de 1 litro. Si se transfiere...
Physics
30.03.2020 18:48
FOR 30 POINTS Suffrage supporters had the greatest success in...
History
14.12.2022 09:00
CaO(s) + SIO2(s) - CaSiO3(s) Calcium silicate (CaSO3) is used...
Chemistry
08.03.2020 07:54
How can human beings work to combat and reduce the many negative...
Biology
29.05.2023 16:50
I have a two questions.. 1. What is the expression for 5 increased...
Mathematics
25.02.2020 20:08
The only time the word incorrectly isn t spelled incorrectly...
Mathematics
06.02.2020 15:45
Find out answers to questions
from an AI-bot
from an AI-bot
Get full access
Answers






