Chemistry
14.04.2021 21:21
153
326
10
Solved by an expert
The Keq for the equilibrium below is 5.4 × 1013 at 480.0 °C. 2NO (g) + O2 (g) 2NO2
The Keq for the equilibrium below is 5.4 × 1013 at 480.0 °C. 2NO (g) + O2 (g) 2NO2 (g) What is the value of Keq at this temperature for the following reaction? NO2 (g) NO (g) + 1/2 O2 (g)
Show Answers
kamjay2006
5,0(28 marks)
The equilibrium constant for 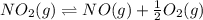 equation is
equation is 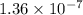
Explanation:
The given chemical equation follows:
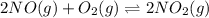
The value of equilibrium constant for the above equation is 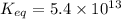
Calculating the equilibrium constant for the given equation:
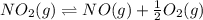
The value of equilibrium constant for the above equation will be:
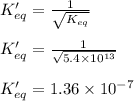
Hence, the equilibrium constant for 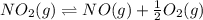 equation is
equation is 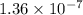
HockeyBlockpk7039
4,8(43 marks)
Um you need some periods. And you spelt it wrong its spelt Kakorrhaphiophobia.
Popular Questions about the subject: Chemistry
What do you read the arrow in a chemical equation as meaning?...
Chemistry
02.02.2021 20:30
Calculate the ph of the aqueous solution of 0.0243 m hno3(aq)....
Chemistry
08.11.2022 12:23
There is approximately 15 milliliters (ml) in 1 tablespoon (tbsp) ? which expression...
Chemistry
31.12.2022 08:50
Considering the principles of KMT, which scenario would result in an increase...
Chemistry
20.09.2020 01:26
Sketch the titration curve for the titration of a generic weak base B with a strong...
Chemistry
06.08.2022 13:09
Unit: Scientific Measurement“One, Two, and More Step Problems” – Wksh #2 1.How...
Chemistry
12.11.2020 03:56
How the moisture present in the sulphur dioxide gas is removed during its lab...
Chemistry
11.10.2021 10:42
A species that has 13 protons and 10 electrons will be...
Chemistry
09.03.2022 00:51
A sample of gas occupies 50.0L at 20.0°C and 515.0mmHg pressure. What is the volume...
Chemistry
20.01.2020 07:38
Hel What is the molarity of a solution where 50.0g of NaCl is added to 2.0L of...
Chemistry
25.03.2023 22:38
New questions by subject
If two of the angles in a triangle are 89° and 30°, how many degrees is the third...
Mathematics
01.06.2021 15:29
The following information is available for Blue Spruce Corp. for the year ended...
Business
02.10.2022 21:20
nder current law, money that you or your employer spend on your health insurance...
Business
14.12.2022 10:23
A brown-eyed man whose father was brown-eyed and whose mother was blue-eyed married...
Biology
20.07.2022 21:57
Fill in the table . What is the limit as x approaches 2?...
Mathematics
22.03.2023 03:43
WHICH OF FOLLOWING IS NOT POSSIBLE? O A. AN OBTUSE ISOSCELES TRIANGLE OB. AN ACUTE...
Mathematics
16.04.2022 13:26
What does nūntiī lībertīs laetīs vīnum optimum ostendēbant mean in latin?...
World Languages
01.11.2020 03:53
Answer the following questions: 1. Which amendment do you think should be ratified...
History
25.03.2023 07:26
Translate the phrase into an algebraic inequality. Use x for each variable. 2....
Mathematics
09.07.2020 18:16
Explain the process that prevents the poles from steadily cooling off and the...
Geography
08.04.2023 07:08
Find out answers to questions
from an AI-bot
from an AI-bot
Get full access
Answers






