The combustion of titanium with oxygen produces titanium dioxide: ti(s) + o2(g)
The heat of reaction for the combustion of a mole of Ti in this calorimeter is - 1.52 × 10^4 KJ/mol.
The equation of the reaction is;
Ti(s) + O2(g) → TiO2(s)
From the available information;
Temperature difference(θ) = 91.60 °C - 25.00 °C = 66.6°C
heat capacity of calorimeter (Cp) = 9.84 kJ/K
Heat absorbed by the calorimeter = cpθ = 9.84 × 10^3 J/K × 66.6°C
= 655.3 KJ
Number of moles of Ti = 2.0600 / 47.867 = 0.0430 mol
Enthalpy of combustion in KJ/mol = -(655.3 KJ)/0.0430 mol
= - 1.52 × 10^4 KJ/mol
Learn more: link
64800 kJ/mol
Explanation:
Heat gained by bomb calorimeter =Q
Heat capacity of bomb calorimeter , C =9.84 kJ/K =
Change in temperature = ΔT= (91.60-25.00) °C = 66.6°C = 66.6 K
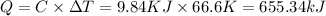
Let the heat released during reaction be q.
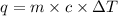
Total heat released during reaction is equal to total heat gained by water and bomb calorimeter.
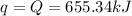
Thus 2.060 g of titanium releases = 655.34 kJ of heat
1 g of titanium releases =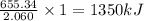 of heat
of heat
1 mole or 48 g of titanium releases =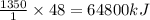 of heat
of heat
Thus heat given off by the burning titanium, in kJ/mol is 64800.
d i think hope that helps
Popular Questions about the subject: Chemistry
New questions by subject
from an AI-bot






