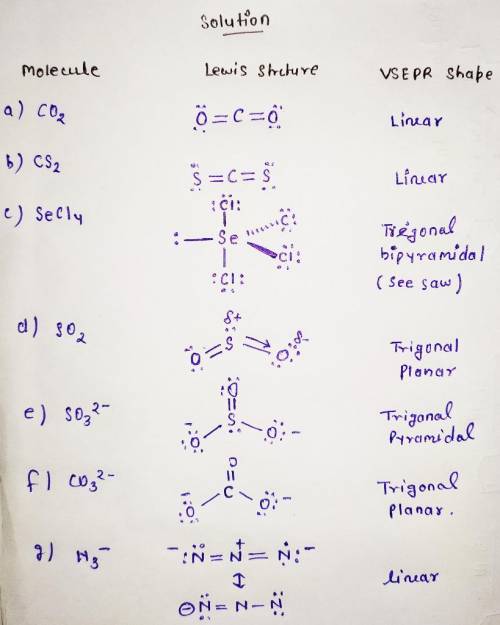
Sketch the simple Lewis dot structures and the predicted VSEPR shapes for each of
shapes for each of the following species. Be sure to clearly indicate
lone pair electrons, multiple bonds (double or triple), and any three-
dimensionality (using dashes and wedges). For any ions, don’t forget
to account for the charge when calculating valence electrons! Only
expand the octet of the central atom when absolutely necessary.
A. CO2
B. CS2
C. SeCL
D. SO2
E. SO32-
F. CO32-
G. N3-
Check the explanation
Explanation:
Kindly check the attached image below to see the step by step explanation to the question above.
Explanation:
Particles A and C are shown as if they are on the same vertical line, which means with the same kinetic energy. Both particle A and C are to the lett of particle B, which means that the formers have a lower kinetic energy than the latter.
Since the likelyhood of a particle to participate in the reaction increases with the kinetic energy, particle B is more likely to participate in the reaction than particles A and C. Hence, the first choice is incorrect.
The graph, although not perfectly symmetrical, does show a bell shape, hence there are many particles will low kinetic energy and many particles with high kinetic energy. You cannot assert that most of the particles of the two gases have high high speeds. Hence, second statement is incorrect, too.
At high values of kinetic energy (toward the right of the curve), the line labeled T₁ is higher than the line labeled T₂, meaning that at T₁ more particles have an elevated kinetic energy than the number of particles that have an elevated kinetic energy at T₂.
On the other hand, at low values of kinetic energy (toward the left of the curve) the line T₂ is higher than the line T₁, meaning that at T₂ more particles have a low kinetic energy than the number of particles that have low kinetic energy at T₁.
Hence, the last two paragraphs are telling that the average kinetic energy of gas particles at T₂ is is lower than the average kinetic energy of gas particles at T₁.
Since the average speed is proportional the the square root of the temperature, the same trend for the average kinetic energy is true for the average speed, and you conclude that the last statement is true: "The average speed of gas particles at T₂ is lower than the average speed of gas particles at T₁".
Since more particles at T₁ have high kinetic energy than the number of particles at T₂ that have a high kinetic energy, more particles of gas at T₁ are likely to participate in the reaction than the gas at T₂, and the third statement is incorrect.
Popular Questions about the subject: Chemistry
New questions by subject
from an AI-bot






