Samarium-146 has a half-life of 103.5 million years. after 1.035 billion years,
0.200 g
0.400 g
20.5 g
103 g
Answer : The correct option is, 0.200 g
Solution :
As we know that the radioactive decays follow first order kinetics.
First we have to calculate the rate constant of a samarium-146.
Formula used :
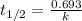
Putting value of 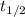 in this formula, we get the rate constant.
in this formula, we get the rate constant.
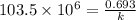
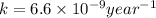
Now we have to calculate the original amount of samarium-146.
The expression for rate law for first order kinetics is given by :
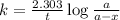
where,
k = rate constant = 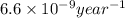
t = time taken for decay process = 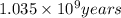
a = initial amount of the samarium-146 = 205 g
a - x = amount left after decay process = ?
Putting values in above equation, we get the value of initial amount of samarium-146.
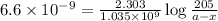
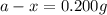
Therefore, the amount left of the samarium-146 is, 0.200 g
Ans: 0.200 g
Given:
Half life of Sm-146 = t1/2 = 103.5 million years
Time period, t = 1.035 billion years = 1035 million years
Original mass of sample, [A]₀ = 205 g
To determine:
Amount of sample after t = 1035 million years
Explanation:
The rate of radio active decay is given as:
water
For many centuries, it was considered that there were only three states of matter: solid, liquid and gas (the three that are present and stable in our world). And water is the substance that best represents them, because it is the only one that exists naturally in the three states.
Explanation: Matter can exist in one of three main states: solid, liquid, or gas.
Solid matter is composed of tightly packed particles. ...
Liquid matter is made of more loosely packed particles. ...
Gaseous matter is composed of particles packed so loosely that it has neither a defined shape nor a defined volume.
Popular Questions about the subject: Chemistry
New questions by subject
from an AI-bot






