Pure nitrobenzene freezes at 5.67 c. when 1.0g of ethanol (c2h6o) is mixed with
The freezing-point depression constant (Kf) of nitrobenzene is 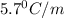
Explanation:
Depression in freezing point is given by:
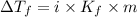
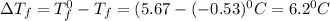 = Depression in freezing point
= Depression in freezing point
i= vant hoff factor = 1 (for non electrolyte nitrobenzene)
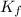 = freezing point constant = ?
= freezing point constant = ?
m= molality
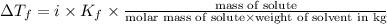
Weight of solvent = 20 g = 0.02 kg
mass of solute (ethanol) = 1.0 g
Molar mass of ethanol = 46 g/mol
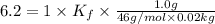
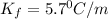
Thus freezing-point depression constant (Kf) of nitrobenzene is 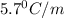
The question is incomplete, the complete question is:
Standardization of a Borax solution (Na2B4O7). A student titrates a 20.00 mL sample of an aqueous borax solution with 1.044 M H2SO4. It takes 2.63 mL of acid to reach the equivalence point. Knowing it takes 1 H2SO4 to neutralize 2 Na2B4O7, what was the concentration of this Borax solution?
The concentration of borax solution is 0.069 M.
Explanation:
To calculate the concentration of borax solution, the formula used is:
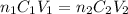 ....(1)
....(1)
where,
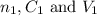 are the n-factor, concentration and volume of sulfuric acid
are the n-factor, concentration and volume of sulfuric acid
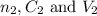 are the n-factor, concentration and volume of borax solution.
are the n-factor, concentration and volume of borax solution.
We are given:
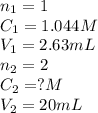
Putting values in equation 1, we get:
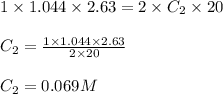
Hence, the concentration of borax solution is 0.069 M.
Popular Questions about the subject: Chemistry
New questions by subject
from an AI-bot






