Chemistry
29.05.2022 11:12
291
494
4
Solved by an expert
Please help with this quiz. A nitrogen-filled balloon has a pressure of 610 torr
Please help with this quiz.
A nitrogen-filled balloon has a pressure of 610 torr at 28°C. What would the pressure
be at 38°C if the volume remained the same?
Show Answers
be at 38°C if the volume remained the same?
pal23
5,0(83 marks)
827.85 torr
Explanation:
Given that,
Pressure, P₁ = 610 torr
Temperature, T₁ = 28°C
We need to find the new pressure if the new temperature is 38°C and if the volume remained the same. Using Gay-lussac's law,
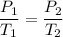
We need to find P₂.
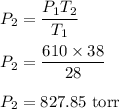
So, the new pressure is 827.85 torr.
Aleja9
4,4(43 marks)
Explanation:
I have explained it in along with the mechanism. If I didn't misinterpret the question then it should be the answer.

Popular Questions about the subject: Chemistry
Why bond angle of h2o is maximum then of2? ?...
Chemistry
02.07.2022 21:39
How does fabric softener affect the flammability of clothes?...
Chemistry
15.12.2020 05:55
Which statement explains the method to adjust the strength of...
Chemistry
14.03.2020 15:27
Calculate the pH of 1.00 L of a buffer that contains 0.105 M...
Chemistry
04.03.2022 11:11
The conductivity of an H2SO4 solution was followed as it was...
Chemistry
02.04.2020 05:25
In order to calculate the mass of an element in a given mass...
Chemistry
16.05.2023 17:30
An intravenous saline drip has 9.8 g of sodium chloride per...
Chemistry
26.11.2020 17:55
40.0g of naoh is dissolved in water. how much heat (in joules)...
Chemistry
15.04.2021 02:43
A1.36-g sample of magnesium nitrate, mg(no3)2, contains mol...
Chemistry
18.03.2021 18:55
How many moles of gas are present in a 1.07 l sample at 1.56...
Chemistry
02.05.2023 13:29
New questions by subject
Angela has a fruit basket with 10 pieces of fruit. It contains...
Mathematics
06.08.2022 19:44
There is a brothers and 3 elderly suspected of being the protector...
English
01.03.2022 03:59
Why did oh PEC increase oil prices in 1973...
History
09.02.2021 06:10
A sample of water contains 5.24 x 1022 molecules. How many moles...
Chemistry
18.05.2021 22:45
A scientist adds 46.3 J of heat to a 13.4g sample of an unknown...
Chemistry
26.05.2021 12:53
Define the term human resources...
Social Studies
02.06.2020 11:58
Read the passage from Darwin s journal What structural adaptation...
Biology
07.08.2020 06:01
Describe three major theories that attempt to explain how the...
History
26.03.2020 16:06
Find out answers to questions
from an AI-bot
from an AI-bot
Get full access
Answers






