List examples of organisms from the movie that represent each of the six kingdoms.
Animalia: Nemo
Plantae: coral
Fungi: algae
Explanation:
I only know 3 sorry
Explanation:
For 1:We are given:
Trial 1:Mass of empty crucible with lid = 26.698 g
Mass of Mg O + crucible + lid = 27.291 g
Actual yield of MgO = (27.291 - 26.698) g = 0.593 g
Trial 2:Mass of empty crucible with lid = 26.687 g
Mass of Mg O + crucible + lid = 27.273 g
Actual yield of MgO = (27.273 - 26.687) g = 0.586 g
For 2:To calculate the theoretical yield of magnesium oxide, we first need to find the actual yield of magnesium for both the trials.
Trial 1:Mass of empty crucible with lid = 26.698 g
Mass of Mg + crucible + lid = 27.060 g
Actual yield of Mg = (27.060 - 26.698) g = 0.362 g
Trial 2:Mass of empty crucible with lid = 26.698 g
Mass of Mg + crucible + lid = 27.046 g
Actual yield of Mg = (27.046 - 26.698) g = 0.359 g
Now, we calculate the number of moles of magnesium for both the trials.
Molar mass of magnesium = 24.31 g/mol
Trial 1: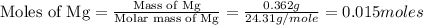
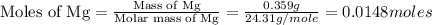
Then, we calculate the number of moles of MgO for both the trial.
The balanced chemical equation for the formation of magnesium oxide follows:
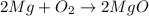
By Stoichiometry of the reaction:
2 moles of Mg metal produces 2 moles of MgO
So, 0.015 moles of Mg will produce = 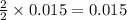 moles of MgO.
moles of MgO.
By Stoichiometry of the reaction:
2 moles of Mg metal produces 2 moles of MgO
So, 0.0148 moles of Mg will produce = 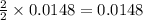 moles of MgO.
moles of MgO.
Now, calculating the theoretical yield of magnesium oxide.
Trail 1: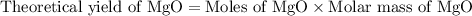
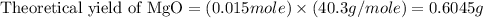
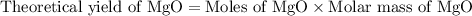
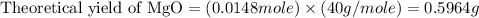
To calculate the percentage yield, we use the formula:
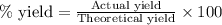
Actual yield of MgO = 0.593g
Theoretical yield of MgO = 0.6045 g
Putting values in above equation, we get:
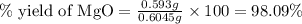
Actual yield of MgO = 0.586 g
Theoretical yield of MgO = 0.5964 g
Putting values in above equation, we get:
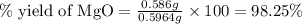
To calculate the average yield, we add on total number of yields and divide it by the number of yields.
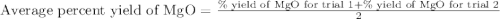
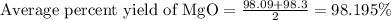
Popular Questions about the subject: Chemistry
New questions by subject
from an AI-bot






