Chemistry
14.04.2023 07:16
182
314
10
Solved by an expert
How does acid (vinegar) react with a metal (paperclip)
How does acid (vinegar) react with a metal (paperclip)
Show Answers
decorajackson01
4,4(10 marks)
I think it can change the paperclip's color.
cutecupcake678
4,4(8 marks)
When 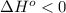 and
and 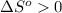
Explanation:
The spontaneity of a reaction can be determined by the sign of the Gibbs free energy change. For a spontaneous reaction, the change in Gibbs free energy should be negative, meaning:
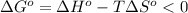
Since the temperature value here corresponds to the absolute temperature, this implies that T > 0 for any T. Therefore, to have a negative difference for any temperature value, the first term, the change in enthalpy, should be negative and the change in entropy should be positive, so that we always subtract a positive number from a negative number. This corresponds to a negative value in 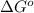 .
.
Popular Questions about the subject: Chemistry
What are potential sources of error in Marta’s experiment? (dont...
Chemistry
24.03.2022 03:29
In coulombs, how much positive charge is there IN a barium nucleus?...
Chemistry
26.12.2021 23:36
The fizz produced when a tablet of alka-seltzer dissolves in...
Chemistry
10.02.2020 22:03
1. as the temperature of the gas in a balloon decreases, which...
Chemistry
13.02.2020 17:51
What information does the atomic mass of an element provide?...
Chemistry
19.10.2022 17:23
What does the arrow mean in a chemical equation?...
Chemistry
30.06.2021 04:38
What is the wavelength of light that has a frequency of 2.85...
Chemistry
27.01.2020 02:42
Which of the following accurately describes semiconductor diodes?...
Chemistry
11.03.2020 15:41
During science class billy used litmus paper to determine the...
Chemistry
05.03.2021 11:24
Determine the type of hybrid orbitals formed by the boron atom...
Chemistry
21.09.2022 22:41
New questions by subject
Original equation ax=b next step 1a (ax)= 1a × b This is an example...
Mathematics
27.02.2021 19:51
Describe two of the possible explanations for the contamination...
Biology
03.10.2020 10:16
The Richmond Park gamekeeper wanted to know how many deer were...
Mathematics
29.03.2023 20:03
Which shows the correct substitution of the values a, b, and...
Mathematics
01.02.2022 00:24
Which is equivalent to (9y2 - 4x)(9y2 + 4x), and what type of...
History
09.05.2021 17:54
Write an equation of the line passing through the given point...
Mathematics
16.11.2020 00:15
What do you Think about Indians and their Prime Minister...
English
01.06.2020 15:53
How does the use of foreshadowing most likely affect the reader?...
English
24.02.2022 01:55
Bernardo: Who’s there? Francisco: Nay, answer me; stand, and...
English
30.01.2021 06:05
What is the text structure of The History of Tornado Forecasting?...
English
08.02.2023 08:20
Find out answers to questions
from an AI-bot
from an AI-bot
Get full access
Answers






