Chemistry
08.12.2021 20:10
238
248
7
Solved by an expert
Given the following reaction, how many liters of H2 gas are needed to form 35.5
Given the following reaction, how many liters of H2 gas are needed to form 35.5 grams of NH3?
N2 + 3H2 -> 2NH3
Show Answers
meramera50
4,7(34 marks)
170.4L
Explanation:
The reaction equation is given as:
N₂ + 3H₂ → 2NH₃
Mass of NH₃ = 35.5g
Problem:
How many liters of H₂ are needed to produce 35.5g of NH₃
Solution:
To solve this problem, let us find the number of moles of NH₃;
Number of moles = 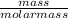
Molar mass of NH₃ = 14 + 3(1) = 17g/mol
So;
Number of moles = 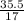 = 5.07mole
= 5.07mole
From the balanced reaction equation:
2 mole of NH₃ will be produced from 3 mole of H₂
5.07mole of NH₃ will produce 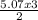 = 7.61mole of H₂
= 7.61mole of H₂
At STP;
1 mole of gas will occupy 22.4L
7.61 mole of H₂ will occupy 7.61 x 22.4 = 170.4L
ValeryGi9733
4,9(44 marks)
This is an example of Avogadro equation
Popular Questions about the subject: Chemistry
A heat engine operates between a high-temperature reservoir at 610 K and...
Chemistry
25.04.2023 20:50
What percent by mass of ammonium sulfate ((NH4)2SO4) is nitrogen(N)?...
Chemistry
27.03.2020 13:03
Select one metal which will displace Sn from a compound and form metallic...
Chemistry
25.06.2023 00:48
Aprokaryote is most likely to use which of the following modes of metabolism...
Chemistry
13.03.2020 04:16
Smaller particles moved by the wind occurs through deflation.t or f....
Chemistry
10.01.2021 16:21
Maki uses 20 gallons of a solution with an unknown ethanol concentration...
Chemistry
15.02.2023 16:51
Which of the following reactions have a positive δsrxn? check all that apply....
Chemistry
02.01.2023 08:59
Ihave a special ideal balloon. this balloon does not exert any pressure...
Chemistry
06.05.2022 22:27
Which is a product of a condensation reaction? 1. o2 2. co2 3. h2 4. h2o...
Chemistry
11.05.2023 14:07
Which element is used for the shiny trim on cars...
Chemistry
26.10.2021 16:34
New questions by subject
You use a coordinate plane to design a kite for a competition. The vertices...
Mathematics
10.04.2023 15:00
Persuading a peace activist to support a war involves: a. changing beliefs...
World Languages
26.03.2020 01:41
Giving 20 points to the right person if your wrong your getting reported...
Mathematics
10.05.2021 05:13
If you are self-employed, you don t have to pay FICA on all your salary,...
Business
01.03.2022 17:52
Over half of the refugees seeking entrance to the United States in 2007...
Geography
31.03.2020 12:23
N the early 1800s, at the time the Missouri territory requested statehood,...
Social Studies
05.10.2020 23:33
Were would you find le sofa? O le garage O la cuisine o la salle de bains...
French
30.01.2020 06:41
A midnight dumper discharges a truck load of industrial waste in a gravel...
Engineering
16.01.2021 14:42
Find the distance between (-8, -1) and (0, 5). Give your answer in simplest...
Mathematics
29.08.2022 21:01
Find out answers to questions
from an AI-bot
from an AI-bot
Get full access
Answers






