Determine the mean effective pressure of an ideal Otto cycle that uses air as the
The mean effective pressure of the ideal Otto Cycle that uses air as the working fluid is; 31.28 psia
We are given;
Initial pressure; P₁ = 14 psia
Initial temperature; T₁ = 60°F = 520 R
Maximum temperature; T₃ = 1500°F = 1960 R
Compression ratio = 9
From tables, the specific heat capacities and constants are;
c_v = 0.171 btu/lbm.R c_p = 0.24 btu/lbm.R R = 0.3704 btu/lbm.R ratio of specific heats; k = 1.4Let us first calculate the initial volume from the formula;
V₁ = RT₁/P₁
V₁ = (0.3704 * 520)/14
V₁ = 13.7577 ft³/lb.m
Compression ratio is; V₁/V₂ = 9
Thus;
V₂ = V₁/9
V₂ = 13.7577/9
V₂ = 1.5286 ft³/lb.m
To get the temperature T₂, we will use the formula;
T₂ = T₁(V₁/V₂)^(k - 1)
T₂ = 520(9)^(1.4 - 1)
T₂ = 1252.277 R
Similarly;
T₄ = T₃/9
T₄ = 1960/(9^(1.4 - 1))
T₄ = 813.87 R
Formula for heat entering and heat exiting are;Q_in = c_v(T₃ - T₂)
Thus;
Q_in = 0.171(1960 - 1252.277)
Q_in = 121.02 btu/lbm
Q_out = c_v(T₄ - T₁)
Q_out = 0.171(813.87 - 520)
Q_out = 50.251 btu/lbm
Net work done is given by;W_net = Q_in - Q_out
W_net = 121.02 - 50.251
W_net = 70.769 btu/lbm
Formula for the mean effective pressure is;mean effective pressure = W_net/(V₁ - V₂)
mean effective pressure = 70.769/(13.7577 - 1.5286)
mean effective pressure = 5.7869 btu.ft³
Converting to psia gives;
Mean effective pressure = 31.28 psia
Read more about compression ratio at; link
The mean effective pressure of the Otto cycle is 31.268 psi
Explanation:
The mean effective pressure is obtained by dividing the work done in the working stroke process of the cycle by the volume of the stroke ar the stroke volume.
In the Otto cycle, therefore, we are to apply an expression for the work done and the volume of the Otto cycle stroke to derive the value of the mean effective pressure as follows.
Here we have the mean effective pressure given by
MEP 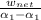
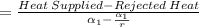
= 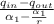
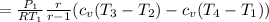
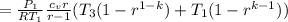
= 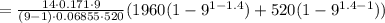
31.268 psi.
simonhhdhfhfb fkfkfb
Popular Questions about the subject: Engineering
New questions by subject
from an AI-bot






