Chemistry
20.03.2022 12:13
287
460
7
Solved by an expert
Calculate the vapor pressure of a solution containing 24.6 g of glycerin (c3h8o3)
Calculate the vapor pressure of a solution containing 24.6 g of glycerin (c3h8o3) in 134 ml of water at 30.0 ∘c. the vapor pressure of pure water at this temperature is 31.8 torr. assume that glycerin is not volatile and dissolves molecularly (i.e., it is not ionic) and use a density of 1.00 g/ml for the water.
Show Answers
marionyallop9205
4,6(42 marks)
Answer : The vapor pressure of a solution is, 30.687 torr
Explanation :
First we have to calculate the moles of glycerin.
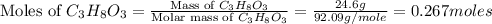
Now we have to calculate the mass of water.
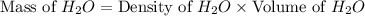
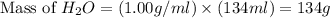
Now we have to calculate the moles of water.
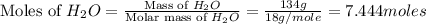
Now we have to calculate the mole fraction of water.
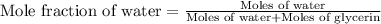
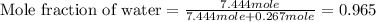
Now we have to calculate the vapor pressure of the solution.
According to the Raoult's law,
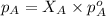
where,
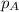 = vapor pressure of solution = ?
= vapor pressure of solution = ?
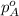 = vapor pressure of pure water= 31.8 torr
= vapor pressure of pure water= 31.8 torr
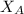 = mole fraction of water = 0.965
= mole fraction of water = 0.965
Now put all the given values in this formula, we get the vapor pressure of solution.
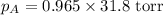
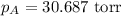
Therefore, the vapor pressure of a solution is, 30.687 torr
andrew8228
4,6(64 marks)
The force would be a balanced force because both of the people are using the same force so it would not move.
Popular Questions about the subject: Chemistry
How is the bond between carbon and hydrogen in methane DIFFERENT...
Chemistry
17.06.2020 04:09
If you use 10 g of CuSO4 and excess NH3, what is the theoretical...
Chemistry
18.03.2020 13:21
Give balanced equation by partial equation method. Steps are...
Chemistry
18.01.2020 10:58
Based on the image, which statement best describes how human...
Chemistry
23.04.2021 18:33
Why must a solution of naoh be standardized using a primary standard,...
Chemistry
18.02.2020 09:17
An unknown substance has a chemical formula A molecule of the...
Chemistry
09.08.2022 00:28
I don’t get it please help Choose an organism. Use credible websites...
Chemistry
27.10.2020 01:55
I saw two birds land on the roof of our porch. One bird had a...
Chemistry
27.06.2020 03:47
Kinetic energy differs from chemical energy in that a.kinetic...
Chemistry
08.04.2023 12:48
What is the oxidation number of iron in fecl3...
Chemistry
04.11.2021 18:25
New questions by subject
A store has 125 scientific calculators. Brand x sells for $20...
Mathematics
20.01.2023 12:25
Wnte a paragraph that states and defends a claim about which...
English
19.04.2022 08:19
If you were a landowner and senator in the Roman Republic, to...
History
04.10.2022 17:13
A party store sold a total of 4,032 balloons since they open...
Mathematics
02.03.2023 23:31
What is the equation relating x and y together? X 1 2 3 4 5 -9...
Mathematics
13.04.2023 07:35
The perimeter of a square (perimeter = 4 times one side) is less...
Mathematics
06.01.2021 07:38
4.00 g of O2 gas are in a sealed, 2.00 L gas canister at 22.0...
Chemistry
17.11.2020 07:23
Complete the sentence with the correct possessive pronoun. That...
English
11.05.2021 23:43
ASAP PLEASE I WILL GIVE BRAINLIEST Click the links to open the...
Geography
17.07.2021 18:37
I don’t know the answer please help...
Mathematics
14.04.2023 15:10
Find out answers to questions
from an AI-bot
from an AI-bot
Get full access
Answers






