Caed for this question. A student reads a barometer in the laboratory and finds
pressure in atmospheres, kilopascals, torrs, pounds per square inch, and pascals.
Hint: 1 atm
101.3 kPa = 760 torr = 760 mm Hg = 14.69 psi = 1.013*10Pa
mm Hg
atm
kPa
torr
psi
Pa
731
Submit Answer
Retry Entire Group
8 more group attempts remaining
0.962 atm.
97.4 kPa.
731 torr.
14.1 psi.
97,434.6 Pa.
Explanation:
Hello.
In this case, given the available factors equaling 1 atm of pressure, each required pressure turns out:
- Atmospheres: 1 atm = 760 mmHg:
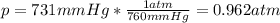
- Kilopascals:: 101.3 kPa = 760 mmHg:
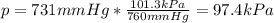
- Torrs: 760 torr = 760 mmHg:
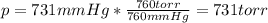
- Pounds per square inch: 14.69 psi = 760 mmHg:
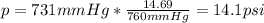
- Pascals: 101300 Pa = 760 mmHg:
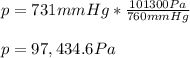
Best regards.
Explanation:
Since Kc is
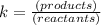
You can insert the Hydrogen and Iodine gas on top, and Hydrogen Iodide in the denominator.
Note: you can only include gases and aqueous species in an equilibrium expression, and all the species in this reaction are gaseous so you're good.
Inserting their molarity at equilibrium into their places, and you can solve. Don't forget to make the coefficient of HI turn into a power.
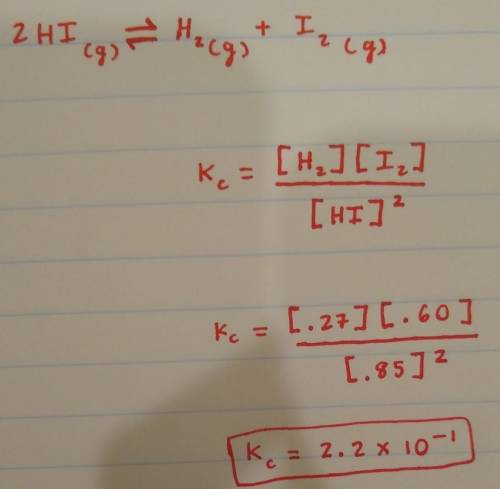
Popular Questions about the subject: Chemistry
New questions by subject
from an AI-bot






