Chemistry
10.03.2021 16:27
117
269
4
Solved by an expert
Apiece of metal weighing 59.047 g was heated to 100.0 °c and then put it into 100.0
Apiece of metal weighing 59.047 g was heated to 100.0 °c and then put it into 100.0 ml of water (initially at 23.7 °c). the metal and water had final temp of 27.8 °c. what is the specific heat of the metal?
Show Answers
briannianoel876
4,6(62 marks)
The specific heat of metal is 0.403J/g°C
Explanation:
To calculate the mass of water, we use the equation:
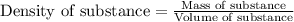
Density of water = 1 g/mL
Volume of water = 100.0 mL
Putting values in above equation, we get:
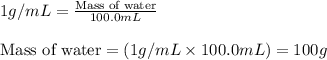
When metal is dipped in water, the amount of heat released by metal will be equal to the amount of heat absorbed by water.
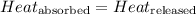
The equation used to calculate heat released or absorbed follows:
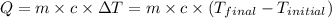
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0020/5842/09236.png) ......(1)
......(1)
where,
q = heat absorbed or released
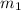 = mass of metal = 59.047 g
= mass of metal = 59.047 g
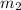 = mass of water = 100 g
= mass of water = 100 g
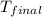 = final temperature = 27.8°C
= final temperature = 27.8°C
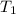 = initial temperature of lead = 100°C
= initial temperature of lead = 100°C
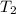 = initial temperature of water = 23.7°C
= initial temperature of water = 23.7°C
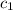 = specific heat of lead = ?
= specific heat of lead = ?
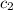 = specific heat of water= 4.186 J/g°C
= specific heat of water= 4.186 J/g°C
Putting values in equation 1, we get:
![59.047\times c_1\times (27.8-100)=-[100\times 4.186\times (27.8-23.7)]](/tpl/images/0020/5842/d5766.png)
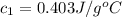
Hence, the specific heat of metal is 0.403J/g°C
keniaguevara32
4,7(33 marks)
Mg (s) + 2 HCl (aq) -> MgCl2 (aq) + H2 (g)
1) Number of mols of HCl, n
n = 6.3 L * 4.5 mol/L = 28.35 mol
2) ratio: 2 mol HCl / 1 mol Mg = 28.35 mol HCl / x mol Mg
x = 28.35 mol HCL * 1 mol Mg / 2 mol HCL = 14.175 mol Mg
3) Convert mol to mass using atomic mass of Mg
14.175 mol Mg * 24.3 g Mg / mol Mg = 344. 45 g
344.45 g
1) Number of mols of HCl, n
n = 6.3 L * 4.5 mol/L = 28.35 mol
2) ratio: 2 mol HCl / 1 mol Mg = 28.35 mol HCl / x mol Mg
x = 28.35 mol HCL * 1 mol Mg / 2 mol HCL = 14.175 mol Mg
3) Convert mol to mass using atomic mass of Mg
14.175 mol Mg * 24.3 g Mg / mol Mg = 344. 45 g
344.45 g
Popular Questions about the subject: Chemistry
Janice wants to grow flowers in her backyard. She has four types of dirt...
Chemistry
23.11.2021 20:23
Which of the following are in the human brain? A. Hypothalamus B. Pituitary...
Chemistry
06.08.2020 12:22
Groups of students Weighed three different samples of magnesium sulfate....
Chemistry
08.01.2021 11:27
How many atoms of each element are present in...
Chemistry
09.05.2021 09:43
Plzzz How many total atoms of oxygen are present in the molecules represented...
Chemistry
30.01.2020 13:54
Put this equation into word form....
Chemistry
07.12.2022 14:54
15. (05.01 MC) Which of the following is most likely to happen if the cell...
Chemistry
18.11.2020 17:21
How many moles are in 2.1× 10^25 molecules of sucrose? round to the 2nd...
Chemistry
11.07.2021 17:33
Which element would most likely form an ion with a -3 charge...
Chemistry
05.07.2021 09:47
New questions by subject
Which of the following statements BEST explains the influence of stable...
English
26.12.2022 01:54
In the table, record the temperature of each thermometer every 2 minutes...
Chemistry
06.04.2023 06:47
How do we see this in the episode of The Simpsons?...
Social Studies
04.11.2021 22:52
Text structure Can someone plz make a description about donuts for me...
English
04.09.2022 12:51
How many neutrons does the element sodium (Na) contain? a 12 b 11 c 13...
Biology
24.04.2021 23:28
50 points 4 questions 1. Which process represents cellular division in...
Biology
12.05.2021 12:35
Does anybody know who figuresk8r89 is? hes been deleting stuff all day...
Health
11.11.2020 00:43
Excluding the force due to air pressure there is only one force acting...
Physics
23.11.2020 18:47
Draw the line of reflection that reflects quadrilateral A B C D ABCDA,...
Biology
18.12.2022 21:45
Find out answers to questions
from an AI-bot
from an AI-bot
Get full access
Answers






