Chemistry
23.04.2021 23:28
137
499
9
Solved by an expert
A49.3 sample of caco3 was treated with aqueous h2so4, producing calcium sulfate,
A49.3 sample of caco3 was treated with aqueous h2so4, producing calcium sulfate, 3.65 g of water and co2(g). what was the % yield of h2o?
Show Answers
jevanoff
4,8(74 marks)
41.1%
Explanation:
First write the balanced reaction:
CaCO₃ + H₂SO₄ → CaSO₄ + H₂O + CO₂
Now calculate the theoretical yield:
49.3 g CaCO₃ × (1 mol CaCO₃ / 100 g CaCO₃) = 0.493 mol CaCO₃
0.493 mol CaCO₃ × (1 mol H₂O / 1 mol CaCO₃) = 0.493 mol H₂O
0.493 mol H₂O × (18 g H₂O / mol H₂O) = 8.87 g H₂O
Now calculate the % yield:
3.65 g H₂O / 8.87 g H₂O × 100% = 41.1%
brookespradley1
4,7(7 marks)
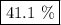
Explanation:
MM: 100.09 18.02
CaCO₃ + H₂SO₄ ⟶ CaSO₄ + H₂O + CO₂
m/g: 49.3 3.65
1. Theoretical yield
(a) Moles of CaCO₃
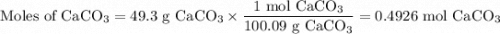
(b) Moles of H₂O
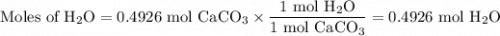
(c) Theoretical mass of H₂O
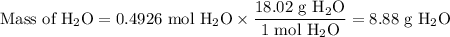
(d) Percent yield
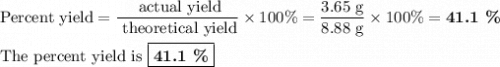
makoa
4,5(10 marks)
Triagonal bypyramid would be the answer in this case.
Popular Questions about the subject: Chemistry
Law of conservation of mass states that all chemicals equations have to...
Chemistry
06.06.2020 21:32
What is the chemical formula of sulfur dioxide? a. so b. so2 c. so3 d....
Chemistry
09.01.2021 00:40
Why is it important to offer evidence to support statements about experiments?...
Chemistry
04.05.2020 05:58
What is the oxidation state for the common action of lithium?...
Chemistry
26.05.2022 00:47
Calculate for reaction shown please...
Chemistry
05.04.2023 10:33
Which of the following chemicals is potentially dangerous? A. ammonium...
Chemistry
04.01.2023 07:25
5. How many moles are in 19 grams of KNO,?...
Chemistry
11.03.2023 11:29
Fe + HCl - Fe + H²SO⁴ (l) - Cu + HCl - Cu + H²SO⁴ (l) - Cu(OH) + HCl -...
Chemistry
23.04.2022 11:31
*best answer will get brainliest* I am testing to see if an egg will float...
Chemistry
11.09.2021 11:28
How many grams of solid barium sulfate form when 21.2 mL of 0.160 M barium...
Chemistry
06.10.2021 11:56
New questions by subject
3/5 page in 3/4 minute (The / mean a fraction )...
Mathematics
21.07.2020 07:12
What do you think could have been done in the years preceding Hurricane...
Geography
16.05.2023 07:08
Question 3 of 10 One of the most stunning features on board the Titanic...
English
10.03.2020 23:21
Determine a rule for i^n where n is any integer and i is an imaginary...
Mathematics
03.09.2020 21:47
The 4Ps model has been challenged because it omits or underemphasizes...
Business
30.08.2020 01:39
to demonstrate an altered perception a student wearing the goggles initially...
Social Studies
18.08.2021 12:15
What types of punishments did those found guilty of being “communists”...
English
27.11.2021 23:44
Find out answers to questions
from an AI-bot
from an AI-bot
Get full access
Answers






