Physics
13.11.2022 13:36
216
269
7
Solved by an expert
A3.50 kg block of lead at 14.4°c comes in contact with a block of copper at 88.4°c.
A3.50 kg block of lead at 14.4°c comes in contact with a block of copper at 88.4°c. they come to equilibrium at 55.9°c. what was the mass of the copper block? (unit=kg)
Show Answers

alsafy383
4,6(93 marks)
1.48 kg
Explanation:
The conservation of energy states that energy cannot be created or destroyed but can be converted from one form to another. Hence, in this case, energy lost by the copper should equal to the energy gained by the lead. Hence,
Energy Lost by Copper = Energy Gained by Lead
mcT = mcT (Bolded is for copper, italicised is for lead)
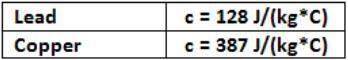
m(387)(88.4 - 55.9) = (3.50)(128)(55.9 - 14.4)
12577.5m = 18592
m = 1.48 kg (3 sf)
(Note: Thermal energy can be calculated using Q = mcT where m is the mass, c is the specific heat capacity and T is the change in temperature)
kcarstensen59070
4,9(87 marks)
B s the correct answer
Popular Questions about the subject: Physics
Enrique is given information about a satellite orbiting earth. r...
Physics
03.10.2022 08:38
What statement best defines work? a.) work is the time it takes to...
Physics
12.11.2021 12:30
True or false, as the frequency of an energy wave increases , so...
Physics
03.03.2023 20:25
URGENT How can a model explain the way in which the moon, the sun,...
Physics
27.07.2020 21:32
4. An automobile gasoline engine is able to do 225 J of useful work...
Physics
14.03.2023 12:01
What is the de Broglie wavelength and speed of an electron accelerated...
Physics
31.03.2023 14:22
Cuál será la temperatura final de un gas si en estado inicial de...
Physics
14.03.2020 00:56
What is the formula that is used to find the velocity of a wave?...
Physics
03.04.2023 15:43
Use the values from PRACTICE IT to help you work this exercise. A...
Physics
28.07.2020 01:47
An observer measures the length (L), width (w), and height (h) of...
Physics
14.04.2021 12:14
New questions by subject
What is the distance between (-6, 8)and (-3, 9)?...
Mathematics
06.05.2023 18:12
What effect would thick cloud cover have cover have on the temperature...
Biology
16.11.2020 22:31
Find ty’s car repair expenses. his car needs a new serpentine belt...
Mathematics
06.04.2021 21:31
Which lines describe the ice wrapping the tree branches? a. as the...
English
21.07.2021 20:41
Saving your report in pdf format a. makes it easy to share electronically...
Business
04.09.2020 13:38
Igot the question wrong two times your answers are wrong you you...
Health
05.03.2022 09:30
Part a: explain why the x-coordinates of the points where the graphs...
Mathematics
10.09.2021 09:38
What was a similarity between convict laborers and sharecroppers...
History
21.04.2023 08:13
Using technological tools to craft reports and proposals a. reduces...
Business
20.05.2020 03:38
What factor is paired with 6 to give 24...
Mathematics
01.01.2020 23:48
Find out answers to questions
from an AI-bot
from an AI-bot
Get full access
Answers






