Chemistry
21.09.2020 19:18
286
367
10
Solved by an expert
A student measures the mass of a sample as 9.64 g. Calculate the percentage error,
A student measures the mass of a sample as 9.64 g. Calculate the percentage error, given that the correct mass is 9.80 g.
Show Answers
jmonee
4,5(51 marks)
The answer is 1.63 %
Explanation:
The percentage error of a certain measurement can be found by using the formula
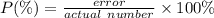
From the question
actual mass = 9.80 g
error = 9.80 - 9.64 = 0.16
We have
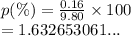
We have the final answer as
1.63 %Hope this helps you
k11kiki
4,5(99 marks)
No it does it, you should a little canola oil. That will make it cook a little faster. Don't add to much though.
Popular Questions about the subject: Chemistry
1. What are the characteristics of a gas?...
Chemistry
04.07.2020 15:59
As human population increases, the amount of human environmental impacts increases...
Chemistry
06.09.2020 22:30
Based on data collected during a laboratory investigation, a student determined...
Chemistry
10.10.2020 03:30
Calculate the following weight and balance given: B.E.W: 1738.93 lbs B.E.W Moment:...
Chemistry
07.03.2022 03:31
How many particles of HCI are required to form 2.3 g of magnesium chloride? HELP...
Chemistry
10.04.2022 10:17
Upto which element the law of octaves was found to be applicable...
Chemistry
26.01.2021 14:19
3. In the chemical reaction CH,+ 20, - CO,+ 2H,0, there are reactants on the left...
Chemistry
08.02.2023 22:41
Gaseous hydrogen iodide is placed in a closed container at 425°c, where it partially...
Chemistry
10.09.2020 20:33
Aluminum metal reacts with bromine, a red-brown liquid with a noxious odor. the...
Chemistry
31.03.2023 00:02
How much water should be added to 85 ml of 0.45 m hci to reduce the concentration...
Chemistry
25.03.2021 20:46
New questions by subject
If d = 2, e = -3, f = 6, and g = 12, what is the value of (d + g) - (e + f) ?...
Mathematics
25.02.2023 20:58
Question What is the opinion that a writer shares with his or her audience in an...
English
02.07.2020 07:13
Write a short note poem at nature in English...
English
30.08.2021 20:37
Qin Shi Huang used which tactic to unify China? A. Allowing conquered people to...
History
26.03.2020 11:13
A battery operated dvd player uses 12 volts from aa batteries and draws a current...
SAT
04.03.2022 07:12
Maura uses 4 tomatoes to make each bowl of salad. Let s represent the number of...
Mathematics
22.03.2022 11:46
Recently, your school embarked on massive entrepreneurial programmes which exposed...
English
05.07.2020 00:07
How did U.S. policy toward American Indians shift over time? A. From a policy of...
History
13.03.2023 02:54
All isotopes of a given element must have the same...
Chemistry
30.08.2021 11:34
Find out answers to questions
from an AI-bot
from an AI-bot
Get full access
Answers






