Law
09.12.2021 06:34
294
449
6
Solved by an expert
A gas at constant pressure occupies 900 ml at 30°C. What would the volume be at
A gas at constant pressure occupies 900 ml at 30°C. What would the volume be
at 45°C?
Show Answers
at 45°C?
aubreywolfe2584
4,6(14 marks)
1350 mL
Explanation:
Given that,
Volume, V₁ = 900 mL
Temperature, T₁ = 30°C
We need to find the new volume if the temperature is 45°C. The relation between temperature and volume is direct (Charles's law).
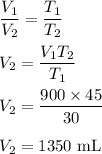
So, the new volume is 1350 mL.
ThGlitchz
4,7(18 marks)
The only Empire to include Saharan salt mine was - Ghana (West Africa)
Popular Questions about the subject: Law
When an inmate is given a reprieve of their sentence, they are...
Law
11.03.2021 18:13
The outcome of the Blackwater case was a very extensive investigation...
Law
30.07.2022 20:10
Please help, being timed! Which pathways in the Transportation...
Law
26.01.2022 22:45
Who makes Local laws? Who makes State laws? Who makes federal...
Law
24.01.2023 06:34
Which case is an example of violating an individual’s Fourth Amendment...
Law
12.01.2023 10:39
What impact did the USA Patriot Act have on protections for online...
Law
12.07.2020 01:04
What is the difference in the lawmaking process at the state and...
Law
15.01.2020 07:25
Copyright protection is designed to protect the privacy of people...
Law
22.08.2021 05:41
In discovery and lawsuits, metadata is used to. confirm or contradict...
Law
03.02.2020 05:58
When the united states’ use of military force is successful in...
Law
03.04.2020 02:14
New questions by subject
1. The volume of a gas is found to occupy one (1) liter of space...
Chemistry
18.12.2021 21:41
PLEASE HELPPP Which statement about converting metric units of...
Mathematics
03.05.2020 07:47
You need to make a liter of 0.225% sodium chloride (NaCl), and...
Mathematics
05.03.2021 02:39
Everybody go check out my You. Tube channel it’s my username and...
Advanced Placement (AP)
14.06.2021 18:08
How the ming dynasty gained power in china?...
History
15.06.2022 11:29
WILL MARK BRAINLIEST LUV YALL At what rate does the rain fall?...
Mathematics
04.10.2021 08:00
#3 - 8.5G Which set of ordered pairs does not show y as a function...
Mathematics
26.01.2020 04:41
The elements of a group have the same number of in their shell....
Chemistry
23.03.2022 00:42
If you re not in high school, please don t answer. I need 5 tips...
English
01.05.2020 22:38
Find out answers to questions
from an AI-bot
from an AI-bot
Get full access
Answers






