A. an endothermic reaction i taking place in a test tube. what would you expect
b. identify each of the following changes as either exothermic or endothermic.
- burning a candle
- lighting a gas stove
- using an instant cold pack
- running a car's engine
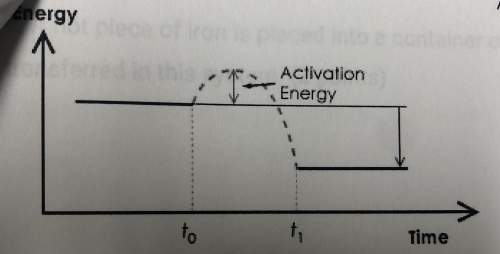
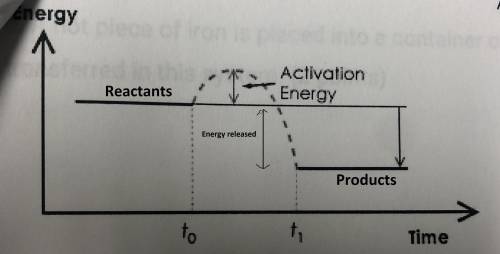
c. on the potential energy diagram below, label the reactants and products. does the diagram shown an endothermic reaction or an exothermic reaction? write one or two sentences explaining your answer.
Explanation:
An endothermic reaction is a type of of chemical reaction in which energy is absorbed from the surrounding. The temperature of the surrounding decreases.
An Exothermic reaction is a type of of chemical reaction in which energy is released into the surrounding. The temperature of the surrounding increases.
a)When an endothermic reaction takes place in test tube the temperature of the surrounding(here the outside wall of the test tube) will decrease which can be felt by touching the outside of the test tube.
b) Burning a candle
,Lighting a gas stove and running a car's engine all are example of exothermic reaction because energy is releases into surroundings. Where as using an instant cold pack is an example endothermic reaction in which energy is absorbed from the surrounding.
c) The diagram is of an exothermic reaction because the energy of the reactants is higher than the energy of the products.The difference in the energies of these two is the energy which was being released on completion of reaction.
a) Endothermic means that the tube will be cold.
b) Candle - exothermic
Lighting a gas stove - exothermic
Using an instant cold pack - endothermic.
Running a car's engine - exothermic.
c) Reactants have higher energy than the products, so it's exothermic cause the difference between energies converted to heat.
1.75
Explanation:
Popular Questions about the subject: Physics
New questions by subject
from an AI-bot






