a 182.4g sample of germanium-66 is left undisturbed for 22.5 hours. At the end of
Approximately 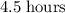 .
.
Explanation:
Calculate the ratio between the mass of this sample after 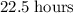 and the initial mass:
and the initial mass:
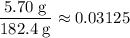 .
.
Let 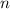 denote the number of half-lives in that
denote the number of half-lives in that 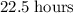 (where
(where 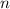 might not necessarily be an integer.) The mass of the sample is supposed to become
might not necessarily be an integer.) The mass of the sample is supposed to become 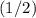 the previous quantity after each half-life. Therefore, if the initial mass of the sample is
the previous quantity after each half-life. Therefore, if the initial mass of the sample is 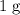 (for example,) the mass of the sample after
(for example,) the mass of the sample after 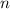 half-lives would be
half-lives would be 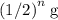 . Regardless of the initial mass, the ratio between the mass of the sample after
. Regardless of the initial mass, the ratio between the mass of the sample after 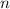 half-lives and the initial mass should be
half-lives and the initial mass should be 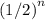 .
.
For this question:
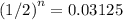 .
.
Take the natural logarithm of both sides of this equation to solve for 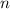 :
:
![\ln \left[{(1/2)}^{n}}\right] = \ln (0.03125)](/tpl/images/1059/5355/e0908.png) .
.
![n\, [\ln(1/2)] = \ln (0.03125)](/tpl/images/1059/5355/97a86.png) .
.
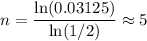 .
.
In other words, there are 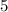 half-lives of this sample in
half-lives of this sample in 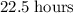 . If the length of each half-life is constant, that length should be
. If the length of each half-life is constant, that length should be 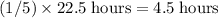 .
.
Explanation:
1. Increasing the concentrations of reactants in a chemical reaction would affect a reaction positively by increasing the number of collisions within a particular period which in turn increases the rate of reaction.
2. The molecules gains energy,which enable them to move faster and collide with the right energy for a reaction to take place.
3. The average kinetic motion of molecules in a solution can be measured by measuring the temperature. Temperature is a measure of the average kinetic energy of molecules in a solution.
Popular Questions about the subject: Chemistry
New questions by subject
from an AI-bot






