3. A 0.5L container of Nitrogen (N) gas at 18 degrees Celsius is heated under constant
The volume increased to 0.64 L on increasing the temperature to boiling point of water.
Explanation:
One of the important laws used for understanding the kinetics of gas particles is Charle's law. It states that the volume occupied by gas molecules will be directly proportional to the Kelvin temperature of the molecules at constant pressure.
Volume ∝ Kelvin Temperature at constant Pressure
So, in this case, the initial volume of the container in which Nitrogen gas is kept is given as 0.5 L and the initial temperature of the Nitrogen gas is said to be 18°C.
Now the container is heated to boiling point of water which is 100°C, then the volume occupied by the gas should increase as per Charle's law.
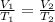
Since here V₁ = 0.5 L
T₁ = 18°C = 18 + 273.15 = 291.15 K
T₂ = 100°C = 100 + 273.15 = 373.15 K
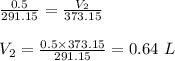
Thus, the volume increased to 0.64 L on increasing the temperature to boiling point of water.
an atom with 3 protons and 4 neutrons is a lithium atom
Popular Questions about the subject: Chemistry
New questions by subject
from an AI-bot






